Clinical Data Management
Build Clinical Studies with Veeva EDC
Build and deploy high quality studies, faster, without custom programming.

Overview
Learn the fundamentals of building studies in Veeva EDC.
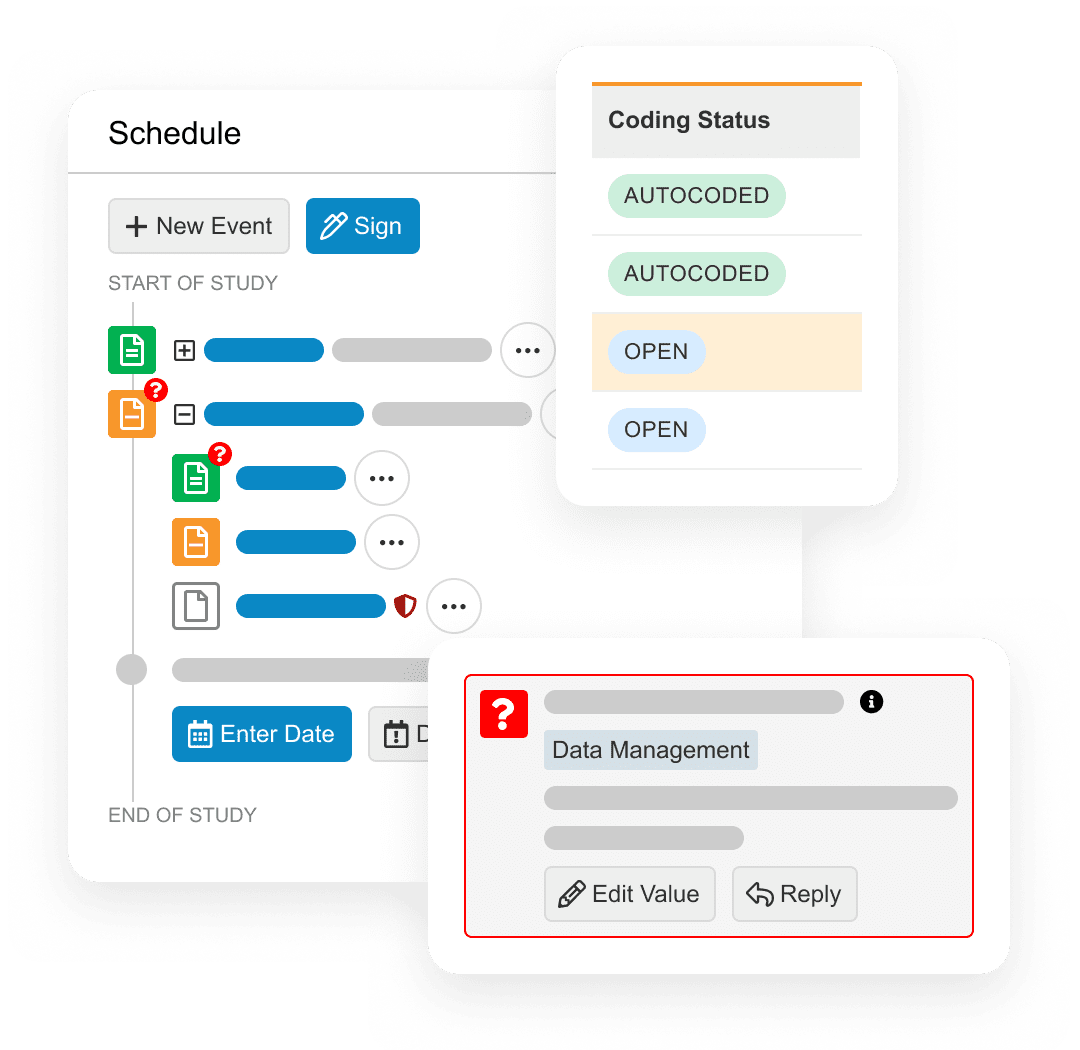
Build and deploy high quality studies, faster, without custom programming.

