Regulatory
Discover Veeva RIM
Learn how to streamline regulatory processes, accelerate submissions & ensure compliant submissions with the unified Veeva RIM suite.
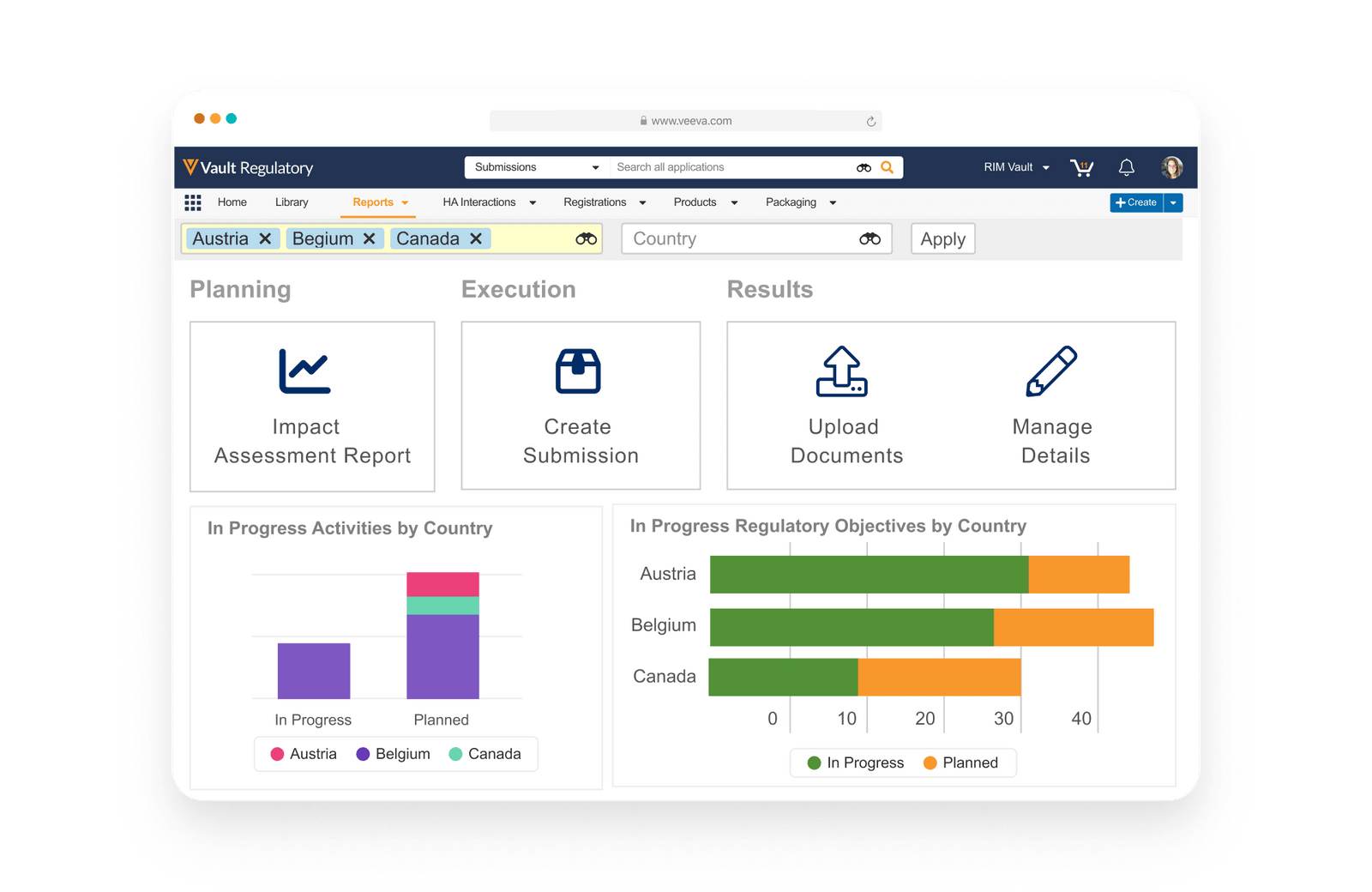
Overview
Streamline global regulatory processes
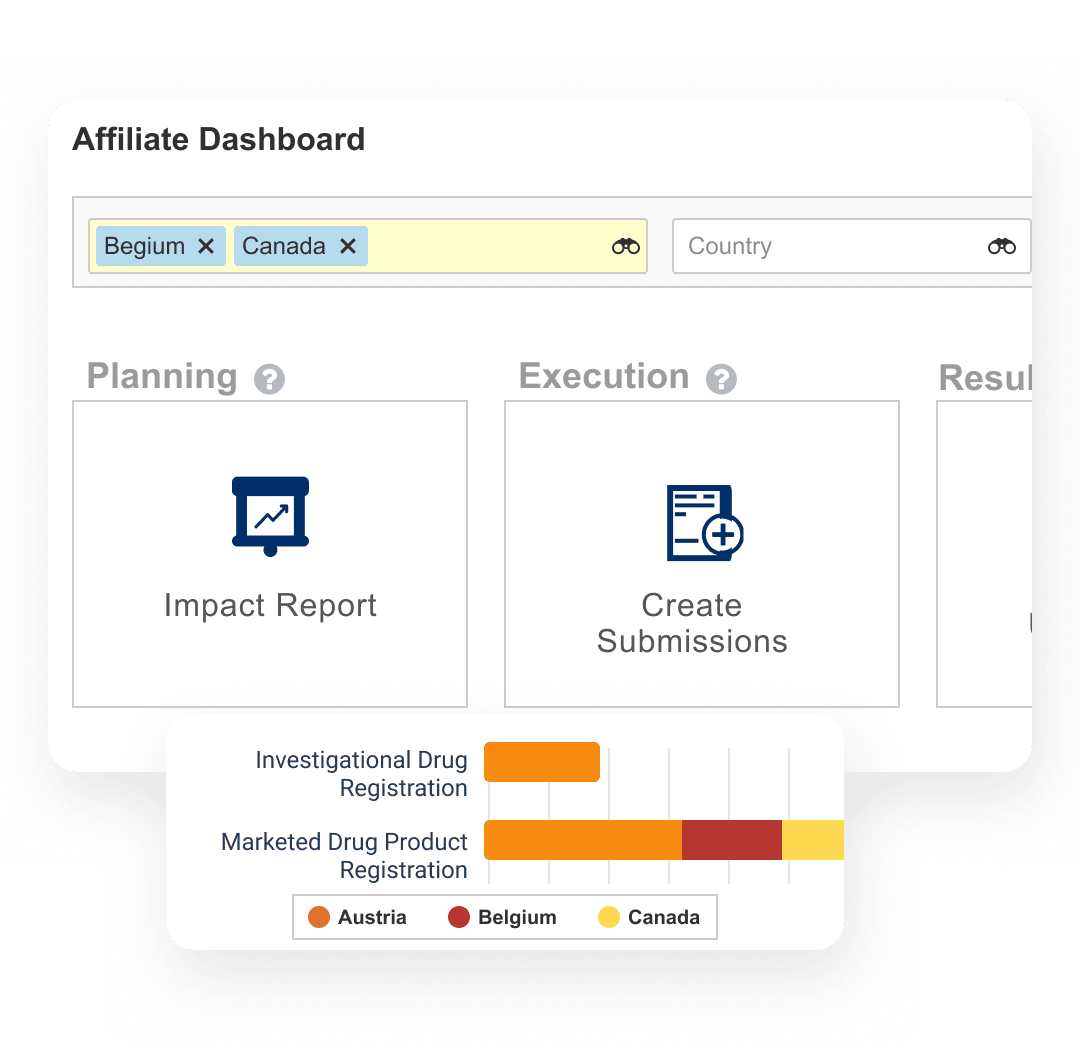
Learn how to streamline regulatory processes, accelerate submissions & ensure compliant submissions with the unified Veeva RIM suite.

